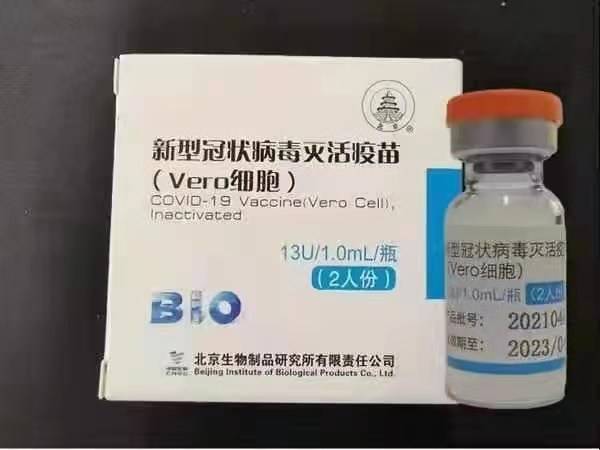
ISinopharm (Beijing): BBIBP-CorV
ISIGABA 1
Isivivinyo
I-ChiCTR2000032459
I-China
ISIGABA 2
2 Ukulingwa
I-NCT04962906
I-Argentina
I-ChiCTR2000032459
I-China
ISIGABA 3
6 Ukulingwa
I-NCT04984408
I-ChiCTR2000034780
I-United Arab Emirates
I-NCT04612972
EPeru
I-NCT04510207
IBahrain, iGibhithe, iJordani, i-United Arab Emirates
I-NCT04560881, BIBP2020003AR
I-Argentina
I-NCT04917523
I-United Arab Emirates
Ukuvunyelwa
Uhlu Lokusebenzisa Okuphuthumayo kwe-WHO 59 Amazwe
Angola 、 Argentina 、 Bahrain 、 Bangladesh 、 Belarus 、 Belize 、 Bolivia (Plurinational State of) 、 Brazil 、 Brunei Darussalam 、 Cambodia 、 Cameroon 、 Chad 、 China 、 Comoros 、 Egypt 、 Equatorial Guinea 、 Gabon 、 Gambia 、 Georgia 、 Guyana 、 Hungary 、 ndonesia 、 Iran (Islamic Republic of) 、 Iraq 、 Jordan 、 Kyrgyzstan 、 I-Republic Yabantu YaseLao
ILebanon 、 IMalaysia iMaldives 、 iMauritania 、 i-auritius 、 Mongolia 、 Montenegro 、 Morocco 、 Mozambique 、 Namibia 、 Nepal 、 Niger 、 North Macedonia 、 Pakistan 、 Paraguay 、 Peru 、 Philippines 、 Republic of the Congo 、 enegal 、 Serbia 、 Seychelles 、 Sierra Leone 、 Solomon Iziqhingi 、 Somalia 、 Sri Lanka 、 Thailand 、 Trinidad and Tobago 、 Tunisia 、 United Arab Emirates 、 Venezuela (Bolivarian Republic of) 、 Viet Nam 、 Zimbabwe
ISinopharm BBIBP-CorV COVID-19 ngumuthi wokugoma ongasebenzi owenziwe ngezinhlayiya zegciwane ezikhuliswe ngamasiko ezingenawo amandla we-pathogenic. Lo mqokelwa ukugoma wasungulwa yiSinopharm Holdings kanye neBeijing Institute of Biological Products.
Umuthi wokugomela weSinopharm BBIBP-CorV usebenza ngokuvumela amasosha omzimba ukuthi akhiqize amasosha omzimba alwa ne-SARS-CoV-2 beta coronavirus. Imithi yokugoma engasebenzi igciwane isetshenziswe amashumi eminyaka, njengokugomela amarabi kanye nomuthi wokugomela i-hepatitis A. Lobu buchwepheshe bokuthuthuka busetshenziswe ngempumelelo emithini yokugoma eminingi eyaziwayo, njengomuthi wokugoma amarabi.
Uhlobo lukaSinopharm lukaSARS-CoV-2 (uhlobo lwe-WIV04 nenombolo yomtapo wolwazi iMN996528) luhlukaniswe nesiguli esibhedlela iJinyintan eWuhan, eChina. Leli gciwane lasakazwa ngokwesiko kulayini onekhono weVero, futhi amandla amakhulu amaseli athelelekile ayengasebenzi ne-β-propiolactone (1: 4000 vol / vol, 2 kuye ku-8 ° C) amahora angama-48. Ngemuva kokucaciswa kwemfucumfucu yeseli kanye ne-ultrafiltration, ukwenziwa kwe-β-propiolactone yesibili kwenziwa ngaphansi kwezimo ezifanayo nokuqala kokungasebenzi. Ngokusho kwe-WHO, lo muthi wokugoma ufakwe ku-0.5 mg we-alum walayishwa emijovweni efakwe u-0.5 ml wamanzi kasawoti oyinyumba oyinyumba ngaphandle kokulondolozwa.
NgoDisemba 31, 2020, i-State Drug Administration yamemezela ukuvunywa komuthi wokuhlola owenziwa yiSinopharm.
NgoMeyi 7, 2021, iWorld Health Organisation yamemezela ukugunyazwa kwalo mgomo. Uhlu lokusetshenziswa kwezimo eziphuthumayo kwe-WHO lwenze amazwe akwazi ukusheshisa izimvume zawo zokulawula ukungenisa nokuphatha umuthi wokugoma i-COVID-19. Iqembu le-WHO Advisory Expert on Strategies Strategies seliphothule ukubuyekeza kwalo muthi wokugoma. Ngokuya ngabo bonke ubufakazi obutholakalayo, i-WHO incoma imithamo emibili yokugoma, amasonto amathathu kuya kwamane ahlukene, kubantu abadala abaneminyaka engu-18 nangaphezulu. Ukusebenza ngokugoma kokulwa nezimpawu ezinesifo nasesibhedlela kulinganiselwa kuma-79% kuyo yonke iminyaka yobudala kuhlangene.
I-American Medical Association ishicilele i- "Randomized Clinical Trial: Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adult" ngoMeyi 26, 2021, iphetha ngokuthi "kulokhu kuhlaziywa kwesikhashana okucacisiwe kwesilingo somtholampilo esingahleliwe, abadala Imithi yokugoma engu-2 engasebenziwe ye-SARS-CoV-2 ehlinzekwe kulokhu kuhlaziywa kwesikhashana okucacisiwe kwezilingo zomtholampilo ezingahleliwe yehlise kakhulu ubungozi be-COVID-19 yezimpawu, futhi izehlakalo ezimbi kakhulu bezingavamile. " Kulesi sigaba 3 sokuhlolwa okungahleliwe kubantu abadala, ukusebenza kwemithi yokugoma eyi-2 engasebenzi ngokuphelele emigomeni yezimpawu ze-COVID-19 kwakungu-72.8% no-78.1%, ngokulandelana. Imishanguzo emi-2 ibinezehlakalo ezimbi kakhulu ezingavamile ezinemvamisa efanayo eqenjini lokulawula i-alum kuphela, futhi iningi lalingahlobene nokugoma. Ukuhlaziywa kokuhlola kutholakale ukuthi imishanguzo emi-2 idale amasosha omzimba alinganiswa angenakulinganiswa, afana nemiphumela yesigaba sesivivinyo se-1/2.
I-WHO SAGE Working Group ishicilele ukubuyekezwa komuthi wokugoma iSinopharm / BBIBP COVID-19 ngoMeyi 10, 2021. Umuthi wokugoma we-GAVID-19 kaGAVI uhlanganisa umuthi wokugomela umuthi wokugoma otshela abasebenzi bezempilo ukuthi ngabe umuthi wokugoma ubulondolozwe kahle futhi awutholakalanga ukushisa ngokweqile. Njengomphumela, umonakalo, i-GAVI ibike ngoMeyi 14, 2021. amalebula ahlakaniphile akhiqizwe yiZebra Technologies futhi enziwa yiTemptime Corporation, aqukethe isiyingi esinesikwele esinombala okhanyayo phakathi, esenziwe ngekhemikhali elingenambala elakha umbala ngokungenakuphindeka ngokuhamba kwesikhathi . Lokhu kuba mnyama ukunikeza inkomba ebonakalayo yokuchayeka kokushisa okukhulayo. Lapho isitsha sesivezwe ekushiseni okungaphezu kobubanzi besitoreji esifanele, isikwele siba mnyama kunesiyingi, okukhombisa ukuthi umuthi wokugoma akusafanele usasetshenziswa.
Inombolo yokubhaliswa kwelabhulali yezidakamizwa ye-National Drug BBIBP-CorV COVID-19: DB15807.